January 2026 – A New Year and Bright Road Ahead!
A New Year and Bright Road Ahead! Dear Partners, I’m beginning my first full year leading Lycored feeling inspired by the significant milestones of 2025: Our...
The next stage of Lycored’s research was to test the effectiveness of Lycoderm™ through a well-controlled, full-scale,
double-blind clinical study. The research, commissioned and funded by Lycored, was conducted in Dusseldorf in Germany and Dundee in the UK. The principal investigator was Professor Jean Krutmann, a leading researcher in the field. It has been published in the peerreviewed scientific journal Skin Pharmacology and Physiology.
Objectives
The aim of the study was to examine the bioavailability,
safety and efficacy of Lycoderm™, and to explore its
potential in enhancing skin resilience and balancing
skin response to UV challenge. Specifically, it sought to
evaluate the effects of Lycoderm™ in a model of UV
induced photodamage in healthy subjects.
Methodology
The study design deliberately included physiological
parameters such as the intensity of erythema that
are easy for consumers to relate to. It also included
mechanistic biomarkers to gain new insights into the
cellular mechanism.
Given that the ingestible skincare space is increasingly
popular with men, the study was strategically designed to
include both genders. One hundred and forty-five healthy
men and women completed a double-blind, randomized,
placebo-controlled parallel group study.
They supplemented for 12 weeks with softgels containing
either Lycoderm™ or a placebo. They were exposed
to controlled local UV radiation at baseline, and again
at the end of supplementation. To gain insights into
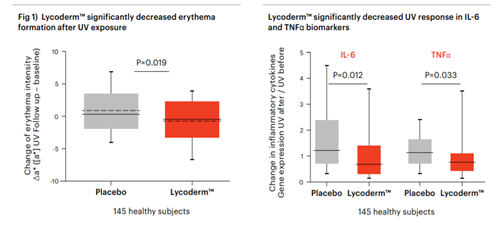
physiological benefits, erythema intensity was measured
following UV exposure. Additionally, to gain insight into mechanism of action at
the cellular level, biopsies from UV-exposed and control
areas were collected and gene expression analysis was
performed. Finally, to verify the causative relationship between
treatment and effect, Lycoderm™’s bioavailability was
evaluated.
Results and conclusions
Lycoderm™ was found to be safe and well tolerated.
A statistically significant decrease in erythema formation
was observed in the group taking the supplement
compared to the placebo group. At the molecular level
there was a reduction in pro-inflammatory cytokines.
This more balanced complexion was reflected in reduced
erythema, or redness of the skin. (Fig 1)
The results provide specific evidence for the mechanism
of action of Lycoderm™, demonstrating a significant effect
on pro-inflammatory cytokines induced by controlled
UV exposure. They also provide definitive proof of the
effect on physiological parameters such as reduction of
the intensity of erythema caused by UV exposure. These
parameters were correlated to a significant increase in
levels of the different carotenoids, supporting a causative
relationship between supplementation with Lycoderm™
and benefits for the skin.

A New Year and Bright Road Ahead! Dear Partners, I’m beginning my first full year leading Lycored feeling inspired by the significant milestones of 2025: Our...

Two of our powerhouse beauty from within solutions — both derived from tomatoes —made the European Food Safety Authority (EFSA) list of novel foods and are officially available for sale...

Breaking news - One ingredient, many solutions! Our new VAS (versatile application solution) technology hit the market November 6th.
Interested in speaking directly with a member of our team?
Click below to get in touch.